A Career Dedicated to Fighting Malaria
-
-
MIT Spectrum
Filed Under
Recommended

Nearly half a million people die each year from malaria, a disease that has been part of the human experience since the dawn of time. With characteristic symptoms of high fever, chills, and weakness, malaria is caused by any of several Plasmodium parasite species, with P. falciparum being responsible for the highest mortality. While the mosquito-borne pathogen has been eradicated in some parts of the world, including the United States and Europe, the developing world continues to suffer.
“An effective vaccine is still highly sought, even after decades of effort; one has recently been approved for clinical use, but it provides only about 30% protection that rapidly wanes,” says Jacquin Niles ’94, PhD ’01, professor of biological engineering and a physician scientist by training. “We have to continue moving forward and doing better.”
To that end, Niles has dedicated his career to fighting malaria.
About 90% of malaria deaths occur in Africa, with children under five years being most commonly afflicted. Raised in the Caribbean, Niles says he is very familiar with the human cost of disease. “I grew up in a context where exposure to different infectious diseases was always a concern; you could see their impact on people and their livelihoods,” says Niles, who is also the director of the MIT Center for Environmental Health Sciences and an associate member of the Broad Institute of MIT and Harvard. “Seeing this impact has been an important motivation for me to pursue research in this field.”
Niles’s career provides a window into the long, uphill journey scientists face in fighting a disease such as Covid-19. “The process of bringing a new drug to market typically has a lead time of about a decade from initial discovery to ensuring that the drug can be safely and efficaciously used in humans,” he points out. He and his team have been working for more than a decade to establish new ways to disrupt the malaria parasite’s life cycle. “The lab spent a lot of time developing the basic technologies we needed to genetically manipulate the parasite so we could more precisely define its vulnerabilities to help propel drug discovery.”
"It’s amazing that a pathogen adopting such a potentially dangerous lifestyle could be among the most successful."
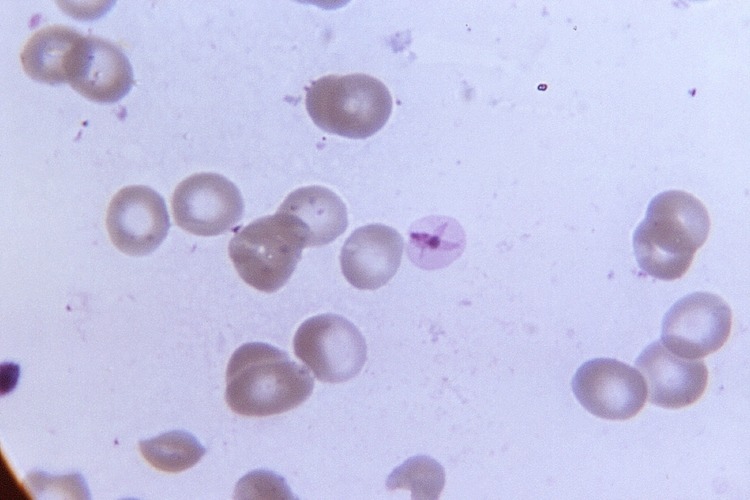
Battling malaria today centers on treatment with antimalarial drugs, but this has drawbacks. “These parasites are resilient,” Niles says. “Resistance to mainstay antimalarial drugs occurs fairly commonly and then spreads around the globe. Some drugs work very well but for a limited time.” Understanding the strategies the parasite uses to survive can provide new insights into possible therapeutics.
Most recently, Niles and his team have focused on how the parasite metabolizes heme, the molecule that makes blood look red. Malaria parasites spend much of their lives in human red blood cells, consuming hemoglobin and releasing heme, which can be toxic but may also be used to support growth. “Our work is revealing aspects of a complex metabolic network the parasite uses to walk a razor’s edge in regulating the balance between beneficial and harmful effects of heme,” he says. “Actually, it’s amazing that a pathogen adopting such a potentially dangerous lifestyle could be among the most successful.”
Disrupting this balance in heme metabolism provides an opportunity for new therapeutics. “We’ve been focused on understanding if there are additional players involved in heme metabolism that would give us new ways to interfere with this process for therapeutic purposes. Targeting this pathway has provided some of the most successful antimalarial drugs used clinically—namely chloroquine and related drugs,” says Niles. Combining drugs with different modes of attacking the pathogen will be critical to fighting drug resistance, Niles adds. This approach has proven successful in fighting other infectious diseases, such as HIV.
While malaria and other killer infectious diseases of the developing world rarely get attention commensurate with their impact on human lives, Niles has a hopeful attitude toward fighting pathogens, and that includes the Covid-19 virus. “The urgency with which resources have been mobilized to combat the Covid-19 pandemic has been wonderful,” he says. “It will likely require a similarly intentional marshaling of resources to eliminate malaria and reduce the human health burden due to neglected diseases. With continued effort and a diversity of ideas focused on these problems, I am optimistic these goals are attainable.”
This story was originally published in the Fall 2020 issue of MIT Spectrum. Microscopic image of the malaria parasite: CDC/Neva Gleason.
Portrait of Jacquin Niles (top): Bryce Vickmark







