
Schneider Lab
origin: 1999 July 29
updated: 2001 February 28
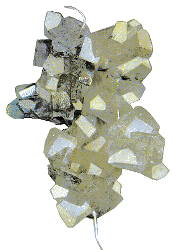
Making rock candy is an example of entropy decrease that you can try in your kitchen. The basic idea is to boil some water and dump in sugar until no more will disolve. You could filter the solution or pour off the liquid into a jar. Then you hang a string into the jar and let it cool.
As the jar cools heat leaves it. So, by the second law of thermodynamics, the entropy of the solution goes down. This allows patterns to form inside the jar, and one observes this as crystals of sugar on the string.
The way to think about what happens is to think at the molecular level. The molecule surfaces are able to fit together. As the heat goes out, the molecules slow down and are able to stick together. Eventually they form a macroscopically visible crystal. If sugar seems too biological for you, use salt (NaCl) or water (H2O) instead. These simple substances occur in the absence of organisms, but upon cooling their solutions form nice crystals.
Living organisms use exactly the same principle to grow. Extremely well characterized examples include:
Example recipies:
![]()

Schneider Lab
origin: 1999 July 29
updated: 2001 February 28
![]()